Determine by liquid
chromatography :
Solution A: 0.05 M monobasic
potassium phosphate
Mobile phase: Methanol and
solution A (60:40). Adjust with 0.1 M phosphoric acid or 1 N sodium hydroxide
to a pH of 5.5, and filter.
Standard stock
solution:
25 mg/ml of Mebendazole ws prepared as follows. Transfer 25 mg of Mebendazole
ws into a 100 ml volumetric flask. Add 10 mL of formic acid, and heat in a
water bath at 50° for 15 min. Shake by mechanical means for 5 min, add 90 mL of
methanol, and allow to cool. Dilute with methanol to volume.
Standard solution: 0.05 mg/mL of Mebendazole
ws in Mobile phase, from Standard stock solution
Sample stock solution: Transfer an
equivalent to 500 mg of mebendazole, from finely powdered Tablets (NLT20), to a 100-mL volumetric flask. Add 50
mL of formic acid, and heat in a water bath at 50° for 15 min. Shake by mechanical
means for 1 h, dilute with water to volume, mix, and filter. Transfer 5.0 mL of
the filtrate to a 100-mL volumetric flask, and dilute with a solution of formic
acid in methanol (1:9) to volume.
for 15 min. Shake by mechanical
means for 1 h, dilute with water to volume, mix, and filter. Transfer 5.0 mL of
the filtrate to a 100-mL volumetric flask, and dilute with a solution of formic
acid in methanol (1:9) to volume.
Sample solution: Nominally 0.05
mg/ml of mebendazole in mobile phase from the sample stock solution. Pass the
solution through a suitable filter of 0.5 µm pore size.
Chromatographic
system
Mode: LC
Detector: UV 247 nm
Precolum: Contains packing L1
Analytical column: 3.9-mm ×
30-cm; packing L1
Temperature: 30°
Flow rate: 1.5 mL/min
Injection size: 15 µL
System
suitability
Sample: Standard
solution
Suitability
requirements
Tailing factor: NMT 2.0
Column efficiency: NLT 2500
theoretical plates
Relative standard
deviation:
NMT 1%
Analysis
Samples: Standard
solution and Sample solution
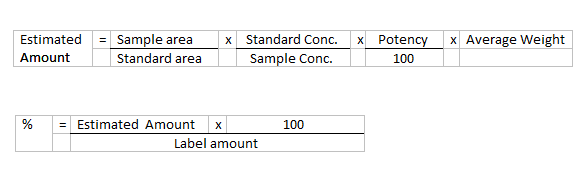
Calculate the
percentage of C16H13N3O3 in the
portion of tablets taken: