Diclofenac Sodium/Capsule
Dissolve 6.8 g of potassium dihydrogen
orthophosphate in 1000 mL of water and adjust the pH to 6.8 with 1M sodium
hydroxide (solution A). To a quantity of the mixed contents of 20
capsules containing 50 mg of Diclofenac Sodium add 10 mL of ethanol (96%)
and mix with the aid of ultrasound for 20 minutes or until completely
dispersed. Add 150 mL of solution A and mix with the aid of ultrasound for a
further 20 minutes or until completely dispersed. Cool to room
temperature, dilute to 250 mL with solution A and shake thoroughly.
Filter the resulting solution and dilute 5 mL to 50 mL with solution A. Prepare
a reference standard in the following manner. Dissolve 50 mg of diclofenac
sodium ws in 10 mL of ethanol (96%) with the aid of ultrasound for
5 minutes. Add 150 mL of solution A and mix with the aid of ultrasound
for a further 5 minutes. Cool to room temperature, dilute to 250 mL with
solution A and shake thoroughly. Dilute 5 mL of the resulting solution to 50 mL
with solution A. Measure the absorbance, of the solutions at 275 nm using
in the reference cell solution A.
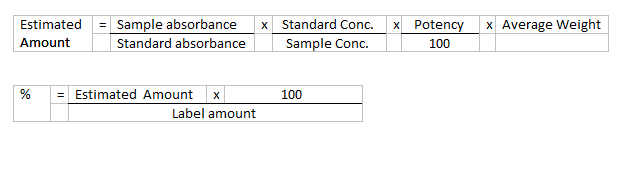
Calculate the content of C14H10Cl2NNaO2
in the capsules using the absorbances at the maximum at 275 nm and the
declared content of C14H10Cl2NNaO2 in
diclofenac sodium ws.
Calculation: